
Chemistry, 20.09.2020 01:01 nandinipp0303
No exemplo a seguir o cátion (Y) do sal interage com o ânion hidróxido (OH-) proveniente da água, e o ânion do sal (X) não interage com o cátion hidrônio (H+) proveniente da água. A hidrólise formada vai gerar um Ph ácido, neutro ou básico? Justifique sua resposta.
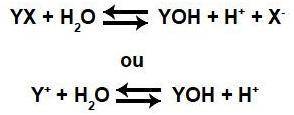

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1
You know the right answer?
No exemplo a seguir o cátion (Y) do sal interage com o ânion hidróxido (OH-) proveniente da água, e...
Questions


Mathematics, 25.09.2020 18:01

Mathematics, 25.09.2020 18:01


Mathematics, 25.09.2020 18:01



History, 25.09.2020 18:01



Mathematics, 25.09.2020 18:01

Mathematics, 25.09.2020 18:01

History, 25.09.2020 18:01


History, 25.09.2020 18:01


Mathematics, 25.09.2020 18:01


Business, 25.09.2020 18:01



