
Chemistry, 20.09.2020 02:01 zanestone12
The vapor pressure of carbon disulfide is 355.6 torr at 25°C. What is the vapor pressure of a solution prepared by dissolving 10.60 g naphthalene (C10H8, Molar Mass = 128.2 g/mol) in 155 mL CS2 liquid (Molar Mass = 76.14 g/mol, density = 1.261 g/mL)? Assume the solution obeys Raoult's law, and treat naphthalene as a nonvolatile solute.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1
You know the right answer?
The vapor pressure of carbon disulfide is 355.6 torr at 25°C. What is the vapor pressure of a soluti...
Questions


Mathematics, 27.09.2019 01:30

Biology, 27.09.2019 01:30




History, 27.09.2019 01:30

Biology, 27.09.2019 01:30


History, 27.09.2019 01:30

Advanced Placement (AP), 27.09.2019 01:30



Mathematics, 27.09.2019 01:30

Health, 27.09.2019 01:30

Biology, 27.09.2019 01:30

Mathematics, 27.09.2019 01:30

Physics, 27.09.2019 01:30

Mathematics, 27.09.2019 01:30

English, 27.09.2019 01:30

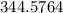 torr
torr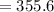 torr
torr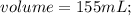
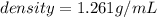
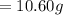
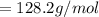
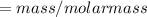
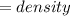 ×
× 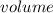
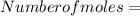
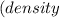 ×
× 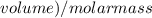
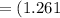 ×
× 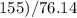
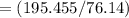
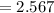 moles of Carbon Disulfide
moles of Carbon Disulfide
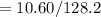
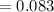
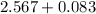
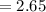 moles
moles
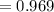
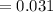
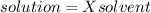 ×
× 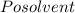
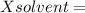 Mole fraction of solvent
Mole fraction of solvent
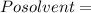 Vapour pressure of the pure solvent
Vapour pressure of the pure solvent
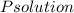
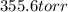
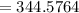 torr
torr

