
Chemistry, 20.09.2020 05:01 daniel9299
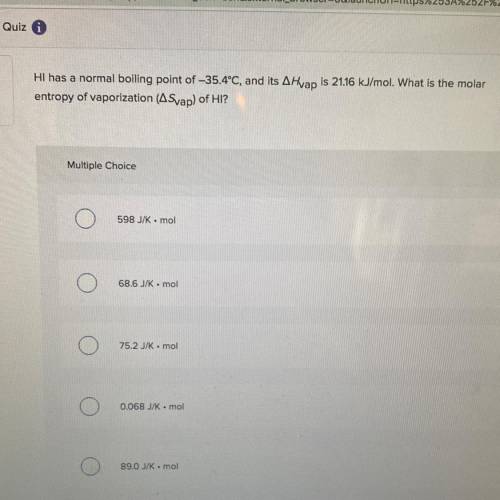
Hi has a normal boiling point of -35.4°C, and its A Hvap is 21.16 kJ/mol. What is the molar
entropy of vaporization (Svap) of HI?
Multiple Choice
598 JK-mol
68.6 JK . mol
752 kmol
0.068 J/K mol
89.0 J/K mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Hi has a normal boiling point of -35.4°C, and its A Hvap is 21.16 kJ/mol. What is the molar
entropy...
Questions

Mathematics, 29.01.2021 05:20


Arts, 29.01.2021 05:20

Mathematics, 29.01.2021 05:20

Mathematics, 29.01.2021 05:20

Mathematics, 29.01.2021 05:20

Chemistry, 29.01.2021 05:20

Mathematics, 29.01.2021 05:20

Mathematics, 29.01.2021 05:20

Mathematics, 29.01.2021 05:20

Mathematics, 29.01.2021 05:20


English, 29.01.2021 05:20




Mathematics, 29.01.2021 05:20

History, 29.01.2021 05:20


Mathematics, 29.01.2021 05:20



