
Chemistry, 20.09.2020 07:01 EricaLSH7624
The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. Calculate the average atomic mass of the element in amu. PLEASE HELP ASAP THANK YOU SO MUCH
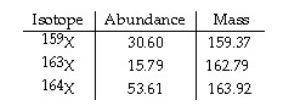

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of...
Questions



History, 05.05.2020 20:59

Mathematics, 05.05.2020 20:59


Mathematics, 05.05.2020 20:59


Biology, 05.05.2020 20:59




Biology, 05.05.2020 21:00



Biology, 05.05.2020 21:00

History, 05.05.2020 21:00

Geography, 05.05.2020 21:00

Chemistry, 05.05.2020 21:00




