
Chemistry, 20.09.2020 07:01 Kjswagout5052
Bismuth oxide reacts with carbon to form bismuth metal: Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g) When 775 g of Bi2O3 reacts with excess carbon, (a) how many moles of Bi form? mol Bi (b) how many grams of CO form? g CO


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Bismuth oxide reacts with carbon to form bismuth metal: Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g) When 775...
Questions



Health, 27.11.2020 21:00










History, 27.11.2020 21:00



World Languages, 27.11.2020 21:00



Mathematics, 27.11.2020 21:00

English, 27.11.2020 21:00

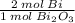 = 3.32632 mol Bi
= 3.32632 mol Bi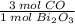 = 4.98948 mol CO
= 4.98948 mol CO

