Chemistry, 20.09.2020 14:01 Bamaboy8804
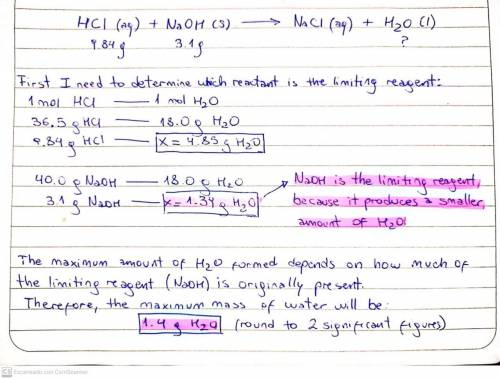
Aqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium chloride NaCl and liquid water H2O. Suppose 9.84 g of hydrochloric acid is mixed with 3.1 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Aqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium...
Questions


Mathematics, 08.01.2020 03:31



English, 08.01.2020 03:31



Mathematics, 08.01.2020 03:31

Mathematics, 08.01.2020 03:31


Mathematics, 08.01.2020 03:31

Mathematics, 08.01.2020 03:31

Mathematics, 08.01.2020 03:31

Mathematics, 08.01.2020 03:31

Biology, 08.01.2020 03:31

Mathematics, 08.01.2020 03:31

Mathematics, 08.01.2020 03:31

Biology, 08.01.2020 03:31


Social Studies, 08.01.2020 03:31