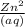or the solution containing Zn2+(aq), n is 2, so the MM/n ratio will be the molar mass of Zn divided by 2: MM/n = (65.39 g/mol) / 2 = 32.70 g/mol Compare this theoretical value with your experimental result. If the plating efficiency is significantly less than 1, say it is 0.55, how would the observed MM/n ratio compare to the theoretical value?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
You know the right answer?
or the solution containing Zn2+(aq), n is 2, so the MM/n ratio will be the molar mass of Zn divided...
Questions



English, 24.06.2019 19:00


History, 24.06.2019 19:00


Mathematics, 24.06.2019 19:00

Mathematics, 24.06.2019 19:00

History, 24.06.2019 19:00

Physics, 24.06.2019 19:00

Physics, 24.06.2019 19:00


Chemistry, 24.06.2019 19:00

History, 24.06.2019 19:00


History, 24.06.2019 19:00

Biology, 24.06.2019 19:00

Mathematics, 24.06.2019 19:00

Mathematics, 24.06.2019 19:00