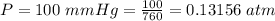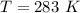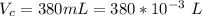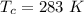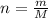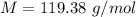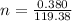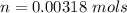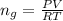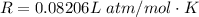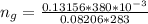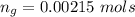Chemistry, 20.09.2020 18:01 juanitarodrigue
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid CHCl3 is placed in a closed, evacuated 380. mL container at a temperature of 283 K.
Assuming that the temperature remains constant, will all of the liquid evaporate? yes/no
What will the pressure in the container be when equilibrium is reached? mm Hg

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

You know the right answer?
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid C...
Questions


Mathematics, 19.01.2022 14:00



History, 19.01.2022 14:00



SAT, 19.01.2022 14:00




Chemistry, 19.01.2022 14:00



SAT, 19.01.2022 14:00

Business, 19.01.2022 14:00