
A common issue in organic chemistry processes is trace contamination. Organic solvents can become contaminated with water. One method of removing the water contamination is to freeze dry the solvent. Unfortunately, not all solvents, like acetonitrile, are compatible with this method. Another method is to add a chemical to react with the water. One such chemical is calcium hydride, which will react to form calcium hydroxide and hydrogen gas. When you checked the chemical inventory, you realized you only had 24.6g of calcium hydride on hand. If you had a 1.5L container of organic solvent that contained 14.0g of water, what will be the outcome of your attempt to dewater the solvent?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
You know the right answer?
A common issue in organic chemistry processes is trace contamination. Organic solvents can become co...
Questions

English, 17.12.2020 14:00



Chemistry, 17.12.2020 14:00




Health, 17.12.2020 14:00

History, 17.12.2020 14:00




Biology, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00

English, 17.12.2020 14:00

Business, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00



English, 17.12.2020 14:00

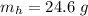

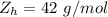

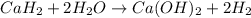

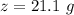 of water
of water 

