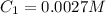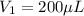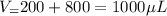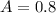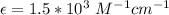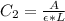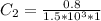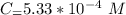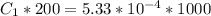Chemistry, 20.09.2020 17:01 haydenamrhein1693
In a laboratory experiment, you are asked to determine the molar concentration of a solution of an unknown compound, X. The solution diluted in with water (200 µL of X + 800 µL of H2O) has an absorbance at 425 nm of 0.8 and a molar extinction coefficient of 1.5 x103 M-1cm-1 at 425 nm. What is the molar concentration of the original solution of X?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
In a laboratory experiment, you are asked to determine the molar concentration of a solution of an u...
Questions



Mathematics, 06.01.2021 07:30


Mathematics, 06.01.2021 07:30


History, 06.01.2021 07:30

Engineering, 06.01.2021 07:30


Social Studies, 06.01.2021 07:30

Mathematics, 06.01.2021 07:30

Advanced Placement (AP), 06.01.2021 07:30


Health, 06.01.2021 07:30

Mathematics, 06.01.2021 07:30


History, 06.01.2021 07:30



Mathematics, 06.01.2021 07:30