
Chemistry, 20.09.2020 17:01 kathrynaveda
The first-order rate constant for the decomposition of N2O5, given below, at 70°C is 6.82 10-3 s-1. Suppose we start with 0.0200 mol of N2O5(g) in a volume of 2.5 L. 2 N2O5(g) → 4 NO2(g) + O2(g) (a) How many moles of N2O5 will remain after 2.5 min? mol (b) How many minutes will it take for the quantity of N2O5 to drop to 0.005 mol? min (c) What is the half-life of N2O5 at 70°C?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 15:00
Does the formation of all chemical bonds is based on sharing of electrons?
Answers: 1
You know the right answer?
The first-order rate constant for the decomposition of N2O5, given below, at 70°C is 6.82 10-3 s-1....
Questions


Chemistry, 25.12.2021 22:40




History, 25.12.2021 22:40

Computers and Technology, 25.12.2021 22:40

History, 25.12.2021 22:40


Chemistry, 25.12.2021 22:40



History, 25.12.2021 22:50

Biology, 25.12.2021 22:50

Mathematics, 25.12.2021 22:50

History, 25.12.2021 22:50

Computers and Technology, 25.12.2021 22:50


English, 25.12.2021 22:50

Social Studies, 25.12.2021 22:50

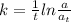
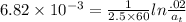
 = 1.023
= 1.023 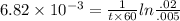
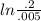 = 3.689
= 3.689

