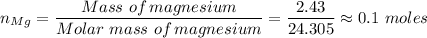Chemistry, 20.09.2020 16:01 msdmdsm1186
You react 2.43 grams of magnesium with oxygen from the air according to the following reaction. The final mass of the product, magnesium oxide, is 4.12 grams. How many grams of oxygen reacted? 2Mg + O2 → 2MgO

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1


Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
You react 2.43 grams of magnesium with oxygen from the air according to the following reaction. The...
Questions

Social Studies, 09.07.2019 10:00



English, 09.07.2019 10:00



Biology, 09.07.2019 10:00


Social Studies, 09.07.2019 10:00

Mathematics, 09.07.2019 10:00


Mathematics, 09.07.2019 10:00


English, 09.07.2019 10:00

Mathematics, 09.07.2019 10:00

Social Studies, 09.07.2019 10:00



Mathematics, 09.07.2019 10:00

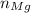 , is given as follows;
, is given as follows;