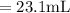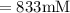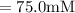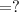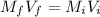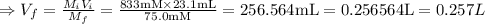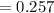A chemist must dilute of aqueous aluminum chloride solution until the concentration falls to . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
A chemist must dilute of aqueous aluminum chloride solution until the concentration falls to . He'll...
Questions

Mathematics, 03.12.2019 23:31





History, 03.12.2019 23:31

Chemistry, 03.12.2019 23:31

History, 03.12.2019 23:31


Chemistry, 03.12.2019 23:31




Mathematics, 03.12.2019 23:31

English, 03.12.2019 23:31

Mathematics, 03.12.2019 23:31

Engineering, 03.12.2019 23:31


History, 03.12.2019 23:31

English, 03.12.2019 23:31