
Chemistry, 20.09.2020 15:01 smithsavannah295
olecular iodine, I2(g), dissociates into iodine atoms at 625 K with a first-order rate constant of 0.271 s-1. (a) What is the half-life for this reaction? s (b) If you start with 0.048 M I2 at this temperature, how much will remain after 5.37 s assuming that the iodine atoms do not recombine to form I2? M

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1
You know the right answer?
olecular iodine, I2(g), dissociates into iodine atoms at 625 K with a first-order rate constant of 0...
Questions

Health, 29.07.2019 13:00

Mathematics, 29.07.2019 13:00

Biology, 29.07.2019 13:00

English, 29.07.2019 13:00

Social Studies, 29.07.2019 13:00

Mathematics, 29.07.2019 13:00

Social Studies, 29.07.2019 13:00

Social Studies, 29.07.2019 13:00

Biology, 29.07.2019 13:00

Health, 29.07.2019 13:00


Computers and Technology, 29.07.2019 13:00


English, 29.07.2019 13:00


Mathematics, 29.07.2019 13:00


Mathematics, 29.07.2019 13:00

Chemistry, 29.07.2019 13:00

Mathematics, 29.07.2019 13:00

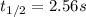
![[I_2]=0.011M](/tpl/images/0771/9802/19391.png)
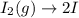
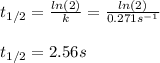
![[I_2]=[I_2]_0exp(-kt)](/tpl/images/0771/9802/e9e96.png)
![[I_2]=0.048Mexp(-0.271s^{-1}*5.37s)\\\\](/tpl/images/0771/9802/4a6e2.png)


