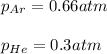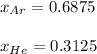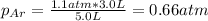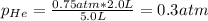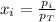Chemistry, 20.09.2020 15:01 noeliaortiz3478
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectively, argon gas at 1.1 atm and helium at 0.75 atm. The containers are initially separated by a valve, and then the valve is opened to connect the two containers. Assume perfect gases and determine the followings.
a. The total pressure of the mixed gases
b. The partial pressure of each gas
c. The mole fraction of each gas

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement about sound is not true? a. air particles travel with sound waves. b. sound waves cannot travel through a vacuum. c. sound waves exist even if no one hears them. d. air particles vibrate along the path of a sound wave.
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectivel...
Questions

Mathematics, 20.05.2020 19:00

Mathematics, 20.05.2020 19:00

Chemistry, 20.05.2020 19:00

English, 20.05.2020 19:00

Computers and Technology, 20.05.2020 19:00



History, 20.05.2020 19:00

Mathematics, 20.05.2020 19:00


Mathematics, 20.05.2020 19:00

Mathematics, 20.05.2020 19:00


History, 20.05.2020 19:00

Biology, 20.05.2020 19:00


Mathematics, 20.05.2020 19:00

English, 20.05.2020 19:00


Mathematics, 20.05.2020 19:00

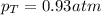 .
.