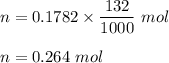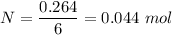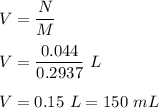Chemistry, 20.09.2020 15:01 genesiloves
Iron in the 2 oxidation state reacts with potassium dichromate to produce Fe3 and Cr3 according to the equation: 6 Fe2 (aq) Cr2O72-(aq) 14 H (aq) <> 6 Fe3 (aq) 2 Cr3 (aq) 7 H2O(l) How many milliliters of 0.2937 M K2Cr2O7 are required to titrate 132.0 mL of 0.1782 M Fe2 solution

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
Iron in the 2 oxidation state reacts with potassium dichromate to produce Fe3 and Cr3 according to t...
Questions

Mathematics, 16.11.2020 21:30

Mathematics, 16.11.2020 21:30

English, 16.11.2020 21:30


Arts, 16.11.2020 21:30


Mathematics, 16.11.2020 21:30

Mathematics, 16.11.2020 21:30


Chemistry, 16.11.2020 21:30


Mathematics, 16.11.2020 21:30

Mathematics, 16.11.2020 21:30

Mathematics, 16.11.2020 21:30

English, 16.11.2020 21:30

English, 16.11.2020 21:30

English, 16.11.2020 21:30

Biology, 16.11.2020 21:30

Mathematics, 16.11.2020 21:30

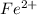 , V = 132 mL .
, V = 132 mL .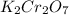 are required to titrate 132.0 mL of 0.1782 M
are required to titrate 132.0 mL of 0.1782 M 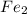 :
: