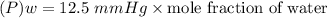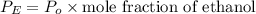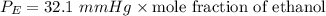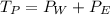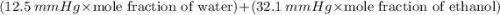Suppose that the mixture in problem 4 is at 15 OC, where the pure vapor pressures are 12.5 mmHg for water and 32.1 mmHg for ethanol. According to Raoult’s Law, the pressure of a component in a solution is equal to its pure vapor pressure times its mole fraction, that is PA = ( ) (XA). Use Raoult’s law to determine the vapor pressure of each component in the solution. Then, add them to find the total vapor pressure. Use significant figures. Show all equations and conversion factors.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Suppose that the mixture in problem 4 is at 15 OC, where the pure vapor pressures are 12.5 mmHg for...
Questions

Biology, 04.01.2021 22:50




History, 04.01.2021 22:50

Computers and Technology, 04.01.2021 22:50

Mathematics, 04.01.2021 22:50


Mathematics, 04.01.2021 22:50


Mathematics, 04.01.2021 23:00




Mathematics, 04.01.2021 23:00



Mathematics, 04.01.2021 23:00

Advanced Placement (AP), 04.01.2021 23:00

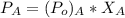
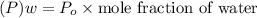
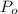 of water = 12.5 mmHg
of water = 12.5 mmHg