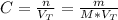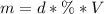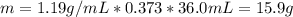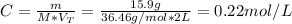Calculate the molarity of a solution made by adding 36.0 mL36.0 mL of concentrated hydrochloric acid ( 37.337.3 % by mass, density 1.19 g/mL1.19 g/mL ) to some water in a volumetric flask, then adding water to the mark to make exactly 2000 mL2000 mL of solution. (It is important to add concentrated acid or base to water, rather than the other way, to minimize splashing and maximize safety.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Calculate the molarity of a solution made by adding 36.0 mL36.0 mL of concentrated hydrochloric acid...
Questions


Mathematics, 05.09.2019 22:10

Physics, 05.09.2019 22:10

Mathematics, 05.09.2019 22:10


Health, 05.09.2019 22:10



Mathematics, 05.09.2019 22:10

English, 05.09.2019 22:10