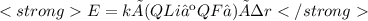Chemistry, 20.09.2020 15:01 briseidam6683
Which factors will result in a stronger ionic bond overall?a. larger ions b. similarity of ionic sizes c. greater absolute charges d. smaller ions

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
Which factors will result in a stronger ionic bond overall?a. larger ions b. similarity of ionic siz...
Questions

Mathematics, 31.01.2021 20:20


Social Studies, 31.01.2021 20:20

History, 31.01.2021 20:20


Physics, 31.01.2021 20:20

History, 31.01.2021 20:20


Mathematics, 31.01.2021 20:20


Mathematics, 31.01.2021 20:20

Biology, 31.01.2021 20:20

Arts, 31.01.2021 20:20

Social Studies, 31.01.2021 20:20


Mathematics, 31.01.2021 20:20

Mathematics, 31.01.2021 20:20

Mathematics, 31.01.2021 20:20

Mathematics, 31.01.2021 20:20

Social Studies, 31.01.2021 20:20