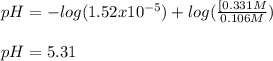A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate the pH of the solution following the addition of 0.061 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The Ka of butanoic acid is 1.52×10−5 .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1


Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1
You know the right answer?
A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate t...
Questions

Physics, 25.06.2019 17:30








Biology, 25.06.2019 17:30






Mathematics, 25.06.2019 17:30





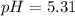
![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/0771/3232/33848.png)
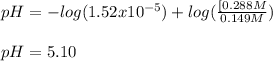
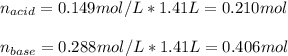
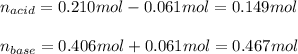
![[acid]=\frac{0.149mol}{1.41L} =0.106M](/tpl/images/0771/3232/f69f4.png)
![[base]=\frac{0.467mol}{1.41L} =0.331M](/tpl/images/0771/3232/b5538.png)