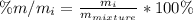Chemistry, 20.09.2020 18:01 kaylaelaine18
A student was provided with a solid sample made up of a mixture of components. Prior to separation, the student measured 2.895 g of the mixture. After separation, the student found the mixture contained the following four components:
Component 1: 1.12g
Component 2: 0.756g
Component 3: 0.254g
Component 4: 0.525g
Which component has the highest mass % for the mixture?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
A student was provided with a solid sample made up of a mixture of components. Prior to separation,...
Questions

Mathematics, 04.07.2019 20:00


Social Studies, 04.07.2019 20:00


Biology, 04.07.2019 20:00

Chemistry, 04.07.2019 20:00




Mathematics, 04.07.2019 20:00

History, 04.07.2019 20:00



Mathematics, 04.07.2019 20:00


English, 04.07.2019 20:00


History, 04.07.2019 20:00