
Chemistry, 20.09.2020 19:01 miguelsanchez1456
An excited ozone molecule, O3*, in the atmosphere can undergo one of the following reactions, O3* → O3 (1) fluorescenceO3* → O + O2 (2) decompositionO3* + M → O3 + M (3) deactivation, where M is an inert molecule, the rate constant for the fluorescence reaction is k1, the rate constant for the decomposition reaction is k2, and the rate constant for the deactivation reaction is k3. Write a simplified expression for the fraction, X, of ozone molecules undergoing deactivation in terms of the rate constants. (Use the following as necessary: k1, k2, k3, cO for [O3*], and cM for [M].)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2


Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3
You know the right answer?
An excited ozone molecule, O3*, in the atmosphere can undergo one of the following reactions, O3* →...
Questions

Biology, 29.05.2021 20:30

Biology, 29.05.2021 20:30

History, 29.05.2021 20:30


Health, 29.05.2021 20:30

Mathematics, 29.05.2021 20:30

Social Studies, 29.05.2021 20:30

Mathematics, 29.05.2021 20:30



Biology, 29.05.2021 20:30








Biology, 29.05.2021 20:30

English, 29.05.2021 20:30

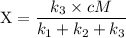
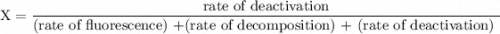
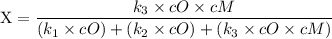
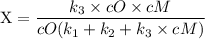
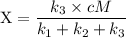 since cM is the concentration of the inert molecule
since cM is the concentration of the inert molecule

