
Chemistry, 20.09.2020 19:01 lucyamine0
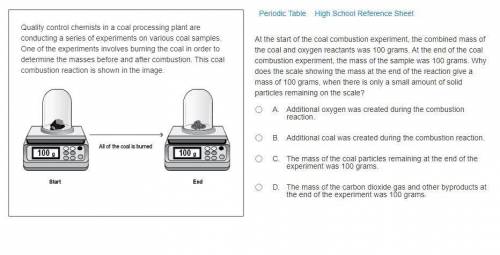
At the start of the coal combustion experiment, the combined mass of the coal and oxygen reactants was 100 grams. At the end of the coal combustion experiment, the mass of the sample was 100 grams. Why does the scale showing the mass at the end of the reaction give a mass of 100 grams, when there is only a small amount of solid particles remaining on the scale?
A. Additional oxygen was created during the combustion reaction.
B. Additional coal was created during the combustion reaction.
C. The mass of the coal particles remaining at the end of the experiment was 100 grams.
D. The mass of the carbon dioxide gas and other byproducts at the end of the experiment was 100 grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
At the start of the coal combustion experiment, the combined mass of the coal and oxygen reactants w...
Questions



History, 30.07.2019 08:00



History, 30.07.2019 08:00

Spanish, 30.07.2019 08:00

Mathematics, 30.07.2019 08:00



Mathematics, 30.07.2019 08:00


Mathematics, 30.07.2019 08:00




History, 30.07.2019 08:00



Mathematics, 30.07.2019 08:00



