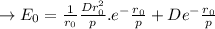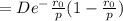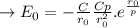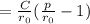Chemistry, 21.09.2020 09:01 mariaveliz2201
The net potential energy EN between two adjacent ions, is sometimes represented by the expression
EN = -(C/r) + D exp(-r/rho)
in which r is the interionic separation and C, D, and rho are constants whose values depend on the specific material.
Required:
a. Derive an expression for the bonding energy E0 in terms of the equilibrium interionic separation r0 and the constants D and rho using the following procedure:
1. Differentiate EN with the respect to r and set the resulting expression equal to zero.
2. Solve for C in terms of D, rho, and ro.
3. Determine the expression for Eo by substitution for C in the equation EN = -(C/r) + D exp(-r/rho)
b. Derive another expression for Eo in terms of ro, C, and rho using a procedure analogous to the one outlined in part (a).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

You know the right answer?
The net potential energy EN between two adjacent ions, is sometimes represented by the expression
E...
Questions



Mathematics, 17.10.2019 11:50

Mathematics, 17.10.2019 11:50

Chemistry, 17.10.2019 11:50


Biology, 17.10.2019 11:50

History, 17.10.2019 11:50


History, 17.10.2019 11:50




Mathematics, 17.10.2019 11:50

History, 17.10.2019 11:50


Computers and Technology, 17.10.2019 11:50

Mathematics, 17.10.2019 11:50


History, 17.10.2019 11:50

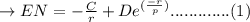
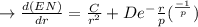
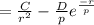
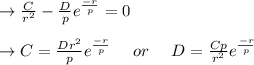
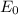 value is replaced by the C value in (1):
value is replaced by the C value in (1):