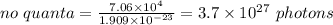Chemistry, 21.09.2020 09:01 mrmendrala
Heating 235 g of water from 22.6°C to 94.4°C in a microwave oven requires 7.06 × 104 J of energy. If the microwave frequency is 2.88 × 1010 s−1, how many quanta are required to supply the 7.06 × 104 J? The value for Planck's constant is 6.63 × 10-34 Jᐧs/quantum. The formula to use is Energy / (Planck's constant x Frequency).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Heating 235 g of water from 22.6°C to 94.4°C in a microwave oven requires 7.06 × 104 J of energy. If...
Questions



History, 25.10.2019 23:43



Biology, 25.10.2019 23:43







History, 25.10.2019 23:43



English, 25.10.2019 23:43


Mathematics, 25.10.2019 23:43

History, 25.10.2019 23:43

Mathematics, 25.10.2019 23:43