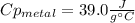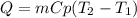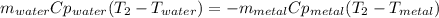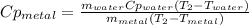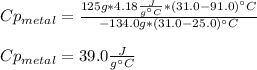Chemistry, 22.09.2020 04:01 jakiyahporter0817
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C. The final temperature of the water is measured at 31.0⁰C. Calculate the specific heat capacity of the unknown metal. Specific Heat of water is 4.18 J/g*C pls answer as quickly as possible

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C...
Questions





Mathematics, 27.01.2020 20:31



Mathematics, 27.01.2020 20:31

English, 27.01.2020 20:31


History, 27.01.2020 20:31


Biology, 27.01.2020 20:31


Business, 27.01.2020 20:31


Chemistry, 27.01.2020 20:31

History, 27.01.2020 20:31

English, 27.01.2020 20:31

Mathematics, 27.01.2020 20:31