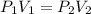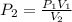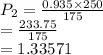Chemistry, 22.09.2020 14:01 jeffreyaxtell4542
A sample of helium gas has a volume of 250.0 mL when it pressure is 0.935 atm. If the
temperature remains constant, what will the pressure of the gas be when it has a volume of
175.0 mL?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
A sample of helium gas has a volume of 250.0 mL when it pressure is 0.935 atm. If the
temperature r...
Questions


Mathematics, 23.03.2021 21:40

Mathematics, 23.03.2021 21:40


Mathematics, 23.03.2021 21:40




Mathematics, 23.03.2021 21:40



Chemistry, 23.03.2021 21:40



Mathematics, 23.03.2021 21:40

Mathematics, 23.03.2021 21:40


Mathematics, 23.03.2021 21:40

Mathematics, 23.03.2021 21:40