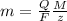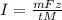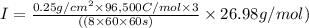The weight loss of an aluminum (Al) alloy corroding in HCI acid was observed to be 0.250 g/cm2 after an 8 h immersion period. What is the corresponding corrosion current density in mA/em2, assuming that all the corrosion is due to the reaction:
Al → Al3+ + 3e
The atomic weight of Al is 26.98 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
The weight loss of an aluminum (Al) alloy corroding in HCI acid was observed to be 0.250 g/cm2 after...
Questions




World Languages, 28.06.2021 15:30


Chemistry, 28.06.2021 15:30

World Languages, 28.06.2021 15:30


English, 28.06.2021 15:30


Biology, 28.06.2021 15:30



Mathematics, 28.06.2021 15:30

History, 28.06.2021 15:40


Mathematics, 28.06.2021 15:40

Social Studies, 28.06.2021 15:40


Physics, 28.06.2021 15:40