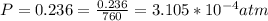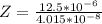Working on-board a research vessel somewhere at sea, you have (carefully) isolated 12.5 micrograms (12.5 ×10–6 g) of what you hope is pure saxitoxin (a non-electrolyte) from a poisonous (and quite cross) puffer fish. You dissolve this sample in 3.10 mL of water and determine that the osmotic pressure of the resulting solution is 0.236 torr at 19ºC (760 torr = 1.00 atm). What is the molar mass of the compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
Working on-board a research vessel somewhere at sea, you have (carefully) isolated 12.5 micrograms (...
Questions


Biology, 01.07.2019 16:50


Mathematics, 01.07.2019 16:50


Physics, 01.07.2019 16:50


Mathematics, 01.07.2019 16:50

History, 01.07.2019 16:50

English, 01.07.2019 16:50




English, 01.07.2019 16:50




Health, 01.07.2019 16:50


Social Studies, 01.07.2019 16:50