
Chemistry, 23.09.2020 14:01 tiwaribianca475
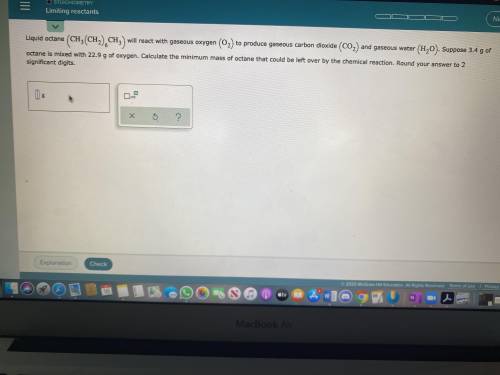
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. Suppose 3.4g of octane is mixed with 22.9g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. Su...
Questions

Mathematics, 24.07.2020 21:01

Biology, 24.07.2020 21:01

Mathematics, 24.07.2020 21:01


Arts, 24.07.2020 21:01


Spanish, 24.07.2020 21:01




English, 24.07.2020 21:01





Mathematics, 24.07.2020 21:01




Mathematics, 24.07.2020 21:01



