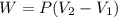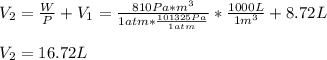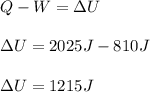Chemistry, 23.09.2020 18:01 natalie2sheffield
A system is composed of 7.14 grams of Ne gas at 298 K and 1 atm. When 2025 joules of heat are added to the system at constant pressure, the resultant expansion causes the system to perform 810 joules of work. Calculate the following. (a) The initial state variables (P, V, T) (b) The final state variables. (c) The change in internal energy for the process. The molecular weight of Ne is 20 and you can assume Ne behaves as an ideal gas. (

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
A system is composed of 7.14 grams of Ne gas at 298 K and 1 atm. When 2025 joules of heat are added...
Questions



Social Studies, 04.12.2020 21:10


Chemistry, 04.12.2020 21:10


Computers and Technology, 04.12.2020 21:10

Computers and Technology, 04.12.2020 21:10

Mathematics, 04.12.2020 21:10

Engineering, 04.12.2020 21:10


History, 04.12.2020 21:10


Mathematics, 04.12.2020 21:10

Physics, 04.12.2020 21:10

Computers and Technology, 04.12.2020 21:10


Biology, 04.12.2020 21:10

Mathematics, 04.12.2020 21:10

Mathematics, 04.12.2020 21:10