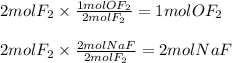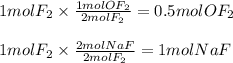A 2 mole sample of F2(g) reacts with excess NaOH(aq) according to the equation above. If the reaction is repeated with excess NaOH(aq) but with 1 mole of F2(g), which of the following is correct?
Group of answer choices
The amount of OF2(g) produced is doubled.
The amount of OF2(g) produced is halved.
The amount of NaF(aq) produced remains the same.
The amount of NaF(aq) produced is doubled.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

You know the right answer?
A 2 mole sample of F2(g) reacts with excess NaOH(aq) according to the equation above. If the reactio...
Questions

Mathematics, 19.08.2020 01:01

Health, 19.08.2020 01:01

Mathematics, 19.08.2020 01:01


Mathematics, 19.08.2020 01:01


Geography, 19.08.2020 01:01

Mathematics, 19.08.2020 01:01


Mathematics, 19.08.2020 01:01


History, 19.08.2020 01:01

Advanced Placement (AP), 19.08.2020 01:01





Social Studies, 19.08.2020 01:01


Health, 19.08.2020 01:01