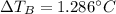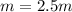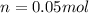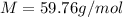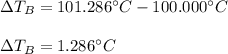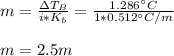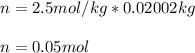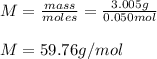Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling point increases from 100.000 OC to 101.286 OC. Determine the TB, molality, moles, and molecular weight for the solute if kb for water is 0.512 OC/m. Report each value using the correct number of significant digits. Refer to Example 1.2 and pages 3-4 in the chapter 1 notes for general chemistry 1 to understand significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3



Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling...
Questions




Health, 21.11.2020 03:00


Mathematics, 21.11.2020 03:00



English, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00


Mathematics, 21.11.2020 03:00

Mathematics, 21.11.2020 03:00






Chemistry, 21.11.2020 03:00