
Chemistry, 24.09.2020 15:01 yungking1329
Calculate the molar volume occupied by 1 mole of N2 using the van der Waals equation in the form of virial expansion at (a) its critical temperature and (b) its Boyle temperature. Assume that the pressure is 10 atm throughout. At what temperature is the gas most perfect? Use the following data: Tc = 126.3 K, a=1.352 L2 atm mol-2, b = 0.0387 L mol-1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
Calculate the molar volume occupied by 1 mole of N2 using the van der Waals equation in the form of...
Questions

Mathematics, 14.01.2021 18:00


History, 14.01.2021 18:00


English, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00


English, 14.01.2021 18:00


English, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00

English, 14.01.2021 18:00


Health, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00

English, 14.01.2021 18:00

 = 1 mole
= 1 mole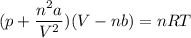
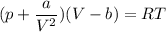
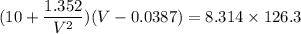
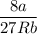
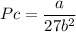
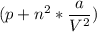 depending on the circumstances.
depending on the circumstances.

