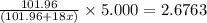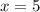Chemistry, 25.09.2020 06:01 witchhunt666
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully in a vacuum oven until no more mass was lost from the sample. After heating, the final weight of the material was 2.6763 g. What was the formula of the hydrated alumina, Al2O3•xH2O? (Enter a whole number for "x") (mol. wt. Al2O3 = 101.96)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully...
Questions

Mathematics, 14.01.2021 15:50



Computers and Technology, 14.01.2021 15:50

History, 14.01.2021 15:50

Mathematics, 14.01.2021 15:50

Mathematics, 14.01.2021 15:50



History, 14.01.2021 15:50

English, 14.01.2021 15:50


Mathematics, 14.01.2021 15:50


Mathematics, 14.01.2021 15:50


Mathematics, 14.01.2021 15:50

Mathematics, 14.01.2021 15:50

History, 14.01.2021 15:50

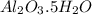
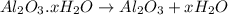
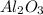 = 101.96 g/mol
= 101.96 g/mol
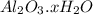 decomposes to give 101.96 g of
decomposes to give 101.96 g of 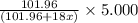 of H_2O
of H_2O