
Chemistry, 25.09.2020 09:01 carlosleblanc26
combustion analysis of a hydrocarbon produced 33.01g CO2 and 13.51g H2O. Calculate the empirical formula for the hydrocarbon

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
combustion analysis of a hydrocarbon produced 33.01g CO2 and 13.51g H2O. Calculate the empirical for...
Questions

History, 23.02.2021 21:00



Advanced Placement (AP), 23.02.2021 21:00




Computers and Technology, 23.02.2021 21:00

History, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00


Mathematics, 23.02.2021 21:00


Physics, 23.02.2021 21:00

History, 23.02.2021 21:00

Biology, 23.02.2021 21:00


Geography, 23.02.2021 21:00

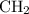 .
.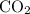 and
and 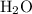 . The
. The  and
and 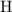 would come from the hydrocarbon, while the
would come from the hydrocarbon, while the  atoms would come from oxygen.
atoms would come from oxygen.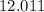 .
.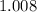 .
.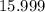 .
.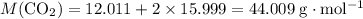 .
.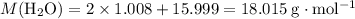
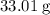 of
of 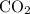 :
: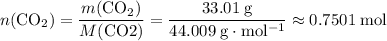 .
.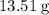 of
of  :
: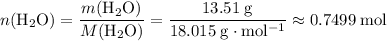 .
.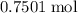 of
of 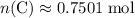 .
.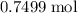 of
of 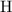 atoms. That is:
atoms. That is: 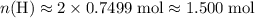 .
.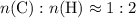 .
. 

