I ONLY HAVE 17 MINS LEFTTT PLEASE HELLLP I WILL REWARD CROWN
...

Chemistry, 25.09.2020 14:01 emilyplays474
I ONLY HAVE 17 MINS LEFTTT PLEASE HELLLP I WILL REWARD CROWN
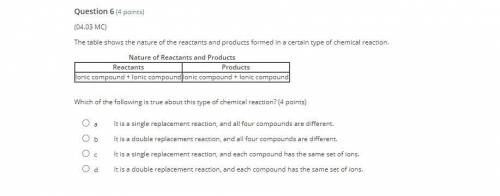

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
Questions


Social Studies, 26.05.2021 23:50




Mathematics, 26.05.2021 23:50

Mathematics, 26.05.2021 23:50


Mathematics, 26.05.2021 23:50

Arts, 26.05.2021 23:50


English, 26.05.2021 23:50


History, 26.05.2021 23:50



Mathematics, 26.05.2021 23:50


English, 26.05.2021 23:50



