
Chemistry, 27.09.2020 15:01 Juniyahodge
ANSWER FAST WILL GIVE BRAINLEIST ALSO 20 points PLEASE ANSWER FAST!!!
Finally, find the coefficients that work in front of each atom or molecule to
balance the equation.
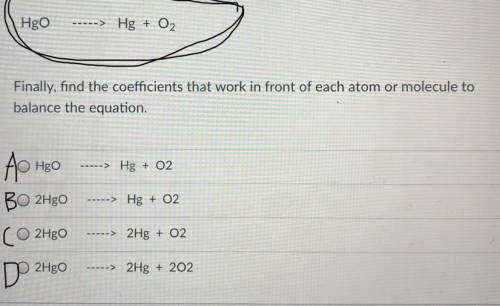

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

You know the right answer?
ANSWER FAST WILL GIVE BRAINLEIST ALSO 20 points PLEASE ANSWER FAST!!!
Finally, find the coefficient...
Questions




Chemistry, 04.05.2020 23:07



Health, 04.05.2020 23:07

Mathematics, 04.05.2020 23:07


Spanish, 04.05.2020 23:07


Mathematics, 04.05.2020 23:07



Biology, 04.05.2020 23:07


Mathematics, 04.05.2020 23:07

History, 04.05.2020 23:07


Mathematics, 04.05.2020 23:07



