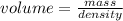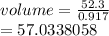Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
You know the right answer?
The density of ice is 0.917 g/cm3. How much volume does 52.3 g of ice occupy? Show your work....
Questions



Mathematics, 03.12.2020 22:50

Physics, 03.12.2020 22:50

History, 03.12.2020 22:50

History, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50


Engineering, 03.12.2020 22:50

Chemistry, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50

French, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50

Social Studies, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50


Advanced Placement (AP), 03.12.2020 22:50

English, 03.12.2020 22:50