
A student is working with four beakers that each contains a clear liquid. Which set of procedures would be best to use to determine whether one of the beakers contains only distilled water?
Group of answer choices
Observe volume, determine mass, observe color, determine pH
Observe odor, determine temperature, observe color, determine boiling point
Observe odor, determine pH, determine density, determine boiling point
Determine mass, observe volume, determine temperature, observe odor

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1


Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
A student is working with four beakers that each contains a clear liquid. Which set of procedures wo...
Questions

Mathematics, 06.11.2020 22:30


Mathematics, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30

Chemistry, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30

Chemistry, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30

Computers and Technology, 06.11.2020 22:30


Mathematics, 06.11.2020 22:30


Mathematics, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30

Health, 06.11.2020 22:30


History, 06.11.2020 22:30

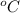 . If the liquid in the beaker ticks all these conditions, then it can be established to be only distilled water.
. If the liquid in the beaker ticks all these conditions, then it can be established to be only distilled water.

