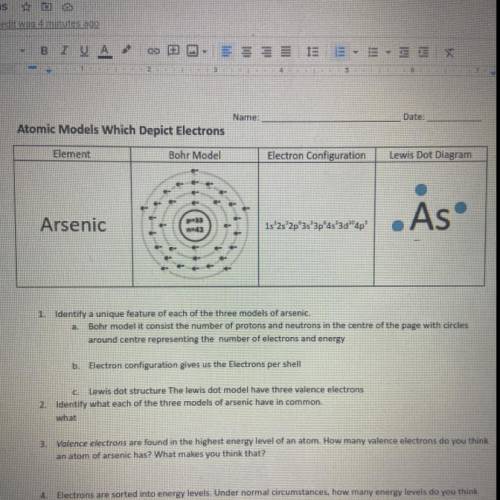
Atomic Models Which Depict Electrons
Element
Bohr Model
Electron Configuration
Le...

Chemistry, 29.09.2020 20:01 queenkendra16
Atomic Models Which Depict Electrons
Element
Bohr Model
Electron Configuration
Lewis Dot Diagram
. As
'
Arsenic
1s 2s 2p 3s 3pas'3d"
ap'
1. Identify a unique feature of each of the three models of arsenie.
Bohr modelit consist the number of protons and neutrons in the centre of the page with circles
around contre representing number of electrons and energy
a
D: Electron configuration gives us the Electrons per shell
Lewis dot structure The lewis dot model have three valence electrons
2. Identify what each of the three models of arsenic have in common
what
Valence electrons are found in the highest energy level of an atom. How many valence electrons do you think
an atom of arsenic has? What makes you think that?
4. Electrons are sorted into energy levels. Under normal circumstances, how many energy levels do you think
contain electrons in an atom of arsenic? What makes you think that?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
Questions


Mathematics, 18.03.2021 03:20



History, 18.03.2021 03:20



Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20


English, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Social Studies, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20



