
Chemistry, 29.09.2020 20:01 cherishofomah04
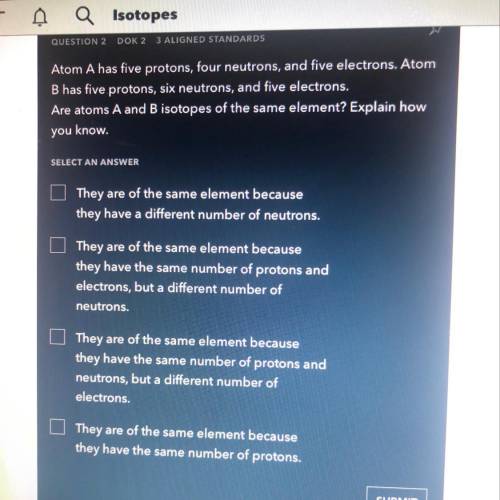
Atom A has five protons, four neutrons, and five electrons. Atom
B has five protons, six neutrons, and five electrons.
Are atoms A and B isotopes of the same element? Explain how
you know.
SELECT AN ANSWER
They are of the same element because
they have a different number of neutrons.
They are of the same element because
they have the same number of protons and
electrons, but a different number of
neutrons.
They are of the same element because
they have the same number of protons and
neutrons, but a different number of
electrons.
They are of the same element because
they have the same number of protons.
SUBMIT

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

You know the right answer?
Atom A has five protons, four neutrons, and five electrons. Atom
B has five protons, six neutrons,...
Questions





Biology, 14.01.2021 17:00



Biology, 14.01.2021 17:00








Mathematics, 14.01.2021 17:00



Computers and Technology, 14.01.2021 17:00

Chemistry, 14.01.2021 17:00



