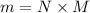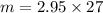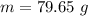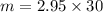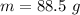Chemistry, 02.10.2020 17:01 trintrin227
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitric oxide, NO.
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Then nitric oxide reacts with methane, CH4.
2NO(g) + 2CH4(g) → 2HCN(g) + 2H2O(g) + H2(g)
When 50.2 g of ammonia and 48.4 g of methane are used, how many grams of hydrogen cyanide can be produced? How many grams of which reactant remain at the end of both reactions? (You may assume that O2 is in excess in the first reaction.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitr...
Questions

Mathematics, 18.10.2019 01:30

Mathematics, 18.10.2019 01:30



History, 18.10.2019 01:30

English, 18.10.2019 01:30

Mathematics, 18.10.2019 01:30

Social Studies, 18.10.2019 01:40



Mathematics, 18.10.2019 01:40

Mathematics, 18.10.2019 01:40

Biology, 18.10.2019 01:40

Mathematics, 18.10.2019 01:40

Social Studies, 18.10.2019 01:40

History, 18.10.2019 01:40


Mathematics, 18.10.2019 01:40


Mathematics, 18.10.2019 01:40

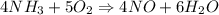
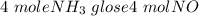
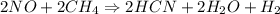
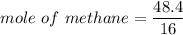
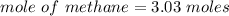
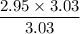 = 2.95 mol HCN
= 2.95 mol HCN