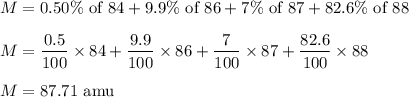Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the h+ concentration for an aqueous solution with poh = 4.01 at 25 ∘c? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of
9.9%), 87...
Questions

English, 11.08.2021 18:30

Biology, 11.08.2021 18:30



English, 11.08.2021 18:30


Physics, 11.08.2021 18:30




Mathematics, 11.08.2021 18:30


History, 11.08.2021 18:30




Health, 11.08.2021 18:30