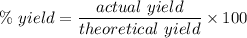Chemistry, 02.10.2020 21:01 briannahernand2
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual percent yield of C in this reaction is 40%. Suppose 10.0 g of A are reacted with excess Compound B, and 6.4 g of Compound C are successfully isolated at the end of the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual per...
Questions

Mathematics, 07.05.2021 14:00

Business, 07.05.2021 14:00

Chemistry, 07.05.2021 14:00

English, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00

Geography, 07.05.2021 14:00

Chemistry, 07.05.2021 14:00



Social Studies, 07.05.2021 14:00



Biology, 07.05.2021 14:00

English, 07.05.2021 14:00

Biology, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00

Chemistry, 07.05.2021 14:00


Mathematics, 07.05.2021 14:00