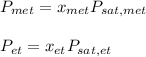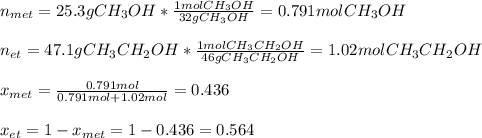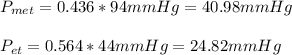Chemistry, 04.10.2020 14:01 melissakm77
ure to answer all parts. The vapor pressure of ethanol (C2H5OH) at 20°C is 44 mmHg, and the vapor pressure of methanol (CH3OH) at the same temperature is 94 mmHg. A mixture of 25.3 g of methanol and 47.1 g of ethanol is prepared and can be assumed to behave as an ideal solution. Calculate the vapor pressure of methanol and ethanol above this solution at 20°C. Be sure to report your answers to the correct number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1
You know the right answer?
ure to answer all parts. The vapor pressure of ethanol (C2H5OH) at 20°C is 44 mmHg, and the vapor pr...
Questions

Mathematics, 21.08.2019 15:10

Social Studies, 21.08.2019 15:10

Physics, 21.08.2019 15:10


Mathematics, 21.08.2019 15:10


Mathematics, 21.08.2019 15:10

Mathematics, 21.08.2019 15:10

Mathematics, 21.08.2019 15:10


Mathematics, 21.08.2019 15:10

History, 21.08.2019 15:10


Arts, 21.08.2019 15:10


Mathematics, 21.08.2019 15:10

Mathematics, 21.08.2019 15:10

Social Studies, 21.08.2019 15:10

Social Studies, 21.08.2019 15:10