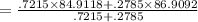Chemistry, 04.10.2020 14:01 Andinojose
Rubidium has two naturally occurring isotopes: 85Rb, with mass 84.9118 amu and natural abundance 72.15%; and 87Rb, with mass 86.9092 amu and natural abundance 27.85%. Calculate the atomic mass of rubidium. View Available Hint(s) Rubidium has two naturally occurring isotopes: 85Rb, with mass 84.9118 amu and natural abundance 72.15%; and 87Rb, with mass 86.9092 amu and natural abundance 27.85%. Calculate the atomic mass of rubidium. 86.3529 amu 84.9118 amu 85.9105 amu 85.4681 amu

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3



Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
Rubidium has two naturally occurring isotopes: 85Rb, with mass 84.9118 amu and natural abundance 72....
Questions






Mathematics, 14.02.2020 04:27

Mathematics, 14.02.2020 04:27

Mathematics, 14.02.2020 04:27

Mathematics, 14.02.2020 04:27


Mathematics, 14.02.2020 04:27




Mathematics, 14.02.2020 04:27


Mathematics, 14.02.2020 04:27



Mathematics, 14.02.2020 04:27