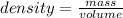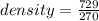Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
8. A shiny, commonly used piece of metal has a mass of 729g and a volume of 270 cm3. what is its den...
Questions

Business, 04.04.2021 23:10


Mathematics, 04.04.2021 23:10

English, 04.04.2021 23:10

English, 04.04.2021 23:10

Mathematics, 04.04.2021 23:10


Chemistry, 04.04.2021 23:10

Mathematics, 04.04.2021 23:10





Mathematics, 04.04.2021 23:10


Mathematics, 04.04.2021 23:10

Health, 04.04.2021 23:10



Mathematics, 04.04.2021 23:20