
Chemistry, 06.10.2020 17:01 Jsquad8879
Group I (the alkali metals) includes lithium (LI), sodium (Na),
and potassium (K). These elements have similar chemical
properties because they have the same
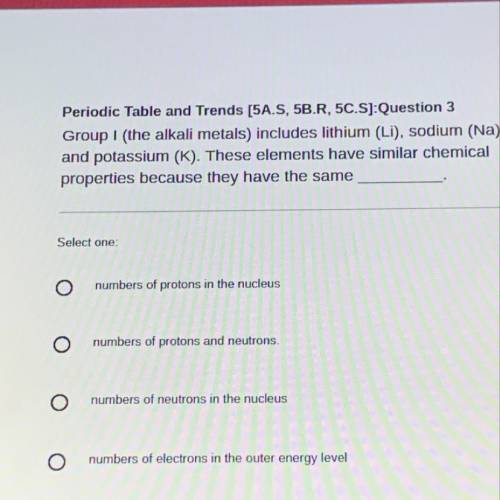

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3
You know the right answer?
Group I (the alkali metals) includes lithium (LI), sodium (Na),
and potassium (K). These elements h...
Questions

Social Studies, 17.07.2019 10:00


Social Studies, 17.07.2019 10:00


Social Studies, 17.07.2019 10:00

Social Studies, 17.07.2019 10:00





Social Studies, 17.07.2019 10:00

Social Studies, 17.07.2019 10:00



Social Studies, 17.07.2019 10:00

History, 17.07.2019 10:00







