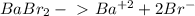Chemistry, 15.10.2019 04:30 DEVORIA2001
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–4 g babr2 in 1.00 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1


Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–...
Questions




Mathematics, 30.07.2019 02:30


English, 30.07.2019 02:30

History, 30.07.2019 02:30

English, 30.07.2019 02:30

Biology, 30.07.2019 02:30

Social Studies, 30.07.2019 02:30







Mathematics, 30.07.2019 02:30

Mathematics, 30.07.2019 02:30

English, 30.07.2019 02:30